AYTU BIOPHARMA (AYTU)·Q2 2026 Earnings Summary
Aytu BioPharma Launches EXXUA, Reports Q2 Revenue of $15.2M as Strategy Pivots to MDD Market
February 3, 2026 · by Fintool AI Agent
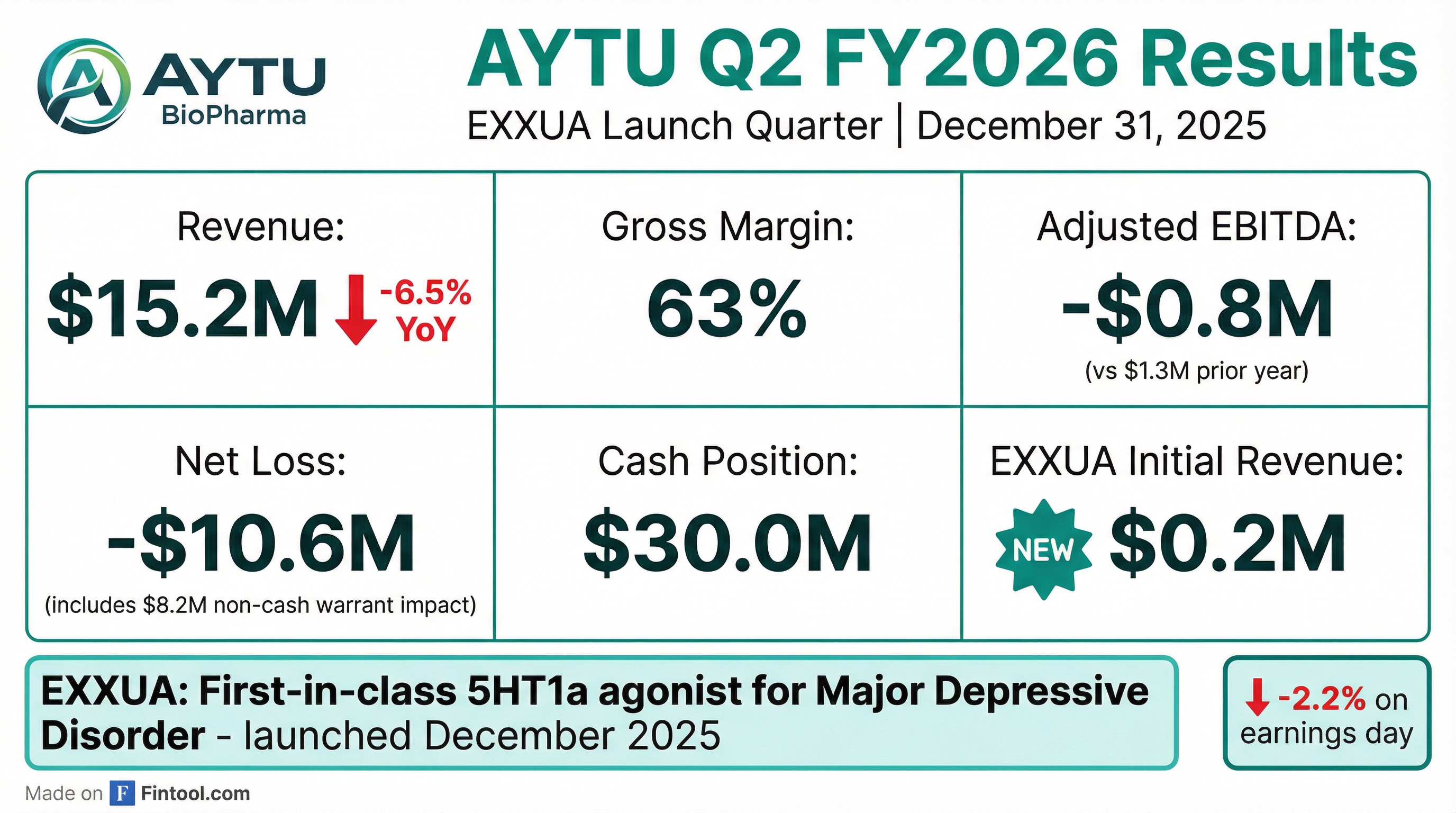
Aytu BioPharma reported fiscal Q2 2026 results that mark a strategic inflection point for the company. Total revenue of $15.2 million declined 6.5% year-over-year as the company intentionally deprioritized its legacy ADHD and Pediatric portfolios to focus resources on the December 2025 commercial launch of EXXUA, a first-in-class treatment for Major Depressive Disorder .
The company recorded a net loss of $10.6 million ($1.05 per share), compared to net income of $0.8 million in Q2 FY2025. However, this loss was heavily impacted by an $8.2 million non-cash derivative warrant liability loss driven by the stock price increase . Adjusted EBITDA was negative $0.8 million, breaking a 10-quarter positive streak, as the company invested in the EXXUA launch .
Did Aytu Beat Earnings?
Aytu BioPharma has limited sell-side analyst coverage, making traditional beat/miss analysis unavailable. The key metrics from Q2 FY2026:
*Cash at June 30, 2025
The year-over-year decline in revenue and profitability was expected as management shifted marketing focus away from legacy products toward EXXUA .
What is EXXUA and Why Does It Matter?
EXXUA represents the most significant product launch in Aytu's history and a fundamental shift in the company's commercial strategy.
Key facts about EXXUA:
- First and only selective serotonin 5HT1a receptor agonist approved by the FDA for Major Depressive Disorder (MDD) in adults
- Addresses a market of approximately 21 million Americans living with MDD
- Targets a $22+ billion US prescription MDD market
- Aytu acquired exclusive US commercialization rights in June 2025
The commercial launch occurred in mid-December 2025, with sales force training completed in mid-January 2026 . Initial stocking revenue was minimal at $0.2 million given the late-quarter timing .
How Did the Stock React?
AYTU shares closed down 2.2% on earnings day (February 3, 2026), falling from $2.71 to $2.62. After-hours trading showed additional weakness with the stock trading at $2.56.
The stock had rallied 26% in December 2025 on the EXXUA launch announcement, rising from $2.06 at the start of December to $2.60 by month-end. The muted reaction to earnings suggests the market is taking a wait-and-see approach on EXXUA commercial traction.
What Changed From Last Quarter?
The key differences from Q1 FY2026:
The sequential revenue improvement reflects the timing of the EXXUA launch in mid-December, though the real EXXUA contribution will become visible in Q3 FY2026 and beyond.
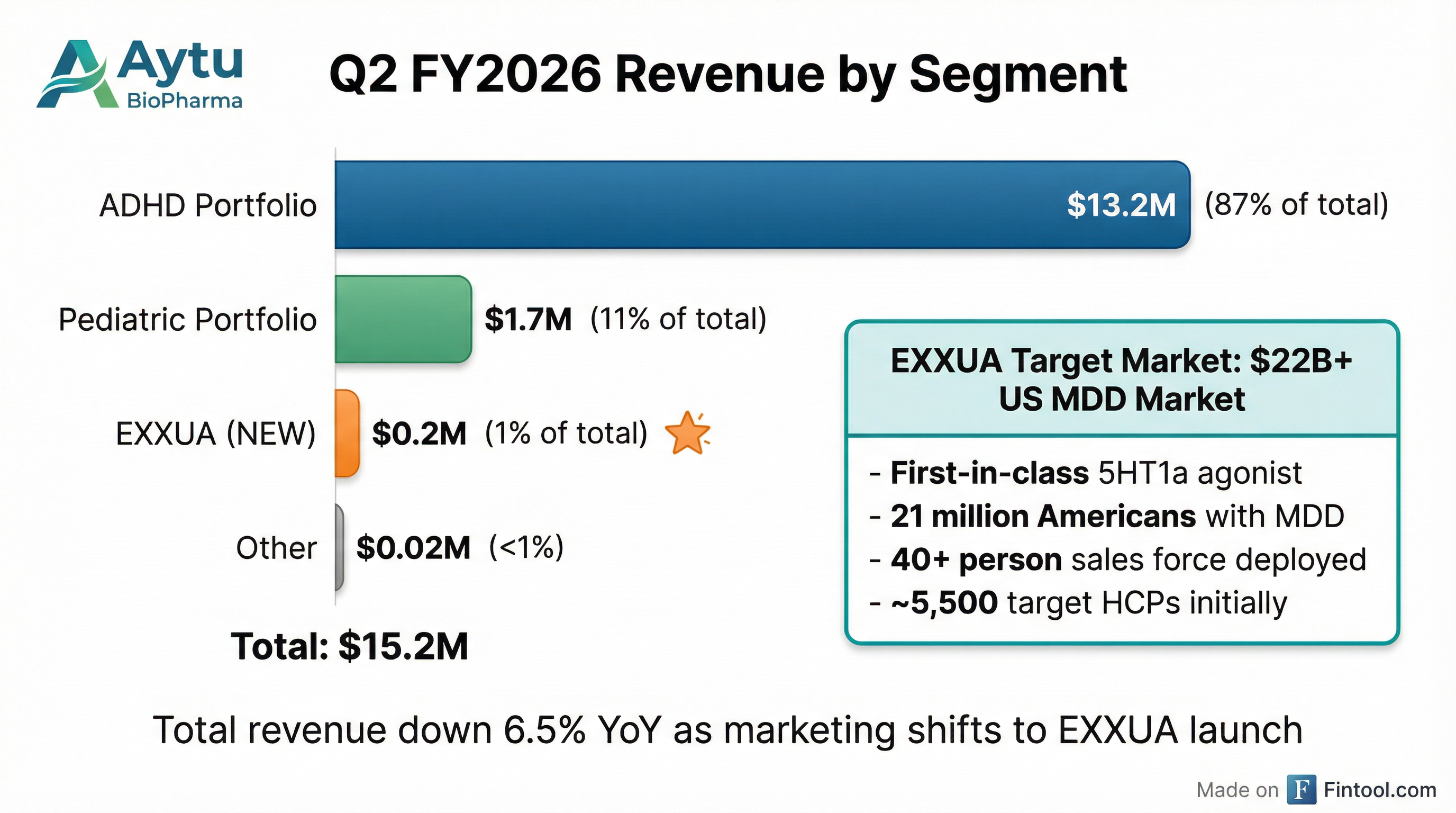
Revenue by Segment
The ADHD portfolio continues to perform "above industry-standard expectations given the evolving dynamics of sales force prioritization toward EXXUA and the recent introduction of generic competition." Management noted approximately 85% of branded ADHD prescriptions flow through the Aytu RxConnect patient access platform .
Generic Competition Update (6-week period ending Jan 16, 2026) :
Management believes RxConnect's "enhanced stickiness" will limit erosion primarily to the ~15% of prescriptions dispensed outside the network. A recent price increase will help offset any script erosion .
What Did Management Say?
CEO Josh Disbrow highlighted the strategic importance of the EXXUA launch:
"This is a truly momentous time for Aytu as we commercially launched EXXUA, the first and only 5HT1a agonist approved by the FDA for the treatment of MDD, representing a new way to treat MDD. Early post-launch indicators have been encouraging."
On execution speed:
"With EXXUA, our team has accomplished in just over six months what often takes years in large pharmaceutical organizations, positioning Aytu at a true inflection point with an opportunity we believe can be transformational for both patients and shareholders."
On early patient feedback:
"The early feedback from patients on EXXUA has been very good. While only a small number of patients have been on EXXUA for a month or longer, they're reporting good tolerability and satisfaction with the product."
On the ADHD portfolio stability:
"Our ADHD portfolio continues to perform above the industry-standard expectations given the evolving dynamics of sales force prioritization toward EXXUA and the recent introduction of generic competition... This continued stability reinforces our long-term conviction in the enhanced stickiness and attractive economic value of the Aytu RxConnect platform."
EXXUA Launch Progress
Key launch milestones achieved:
- ✅ All national distributors and wholesalers fully stocked
- ✅ 40+ person sales force deployed across the US
- ✅ Sales force training completed mid-January 2026
- ✅ 100+ doctors have prescribed EXXUA to date
- ✅ Scripts written from 27 states (including states without sales reps)
- ✅ First refills already occurring (patients on therapy 30+ days)
- ✅ Key Opinion Leader (KOL) network actively expanding
Commercial strategy details:
- Territory coverage: ~140 million total MDD prescriptions, 18.5 million target HCP prescriptions
- Initial focus: ~5,500 target healthcare practitioners
- Patient access: Leveraging Aytu RxConnect platform with ~60% commercial insurance coverage for MDD patients
- Expansion plans: Virtual sales team and rolling Contract Sales Organization model to scale
- Patient out-of-pocket capped at $50/prescription for commercially insured patients
Weather Impact: Management noted that severe winter weather across most of the country disrupted the first full month of launch, with "two solid weeks of weather affecting a huge chunk of our territories." Despite this, the company is encouraged by early traction .
Balance Sheet & Liquidity
Cash burn of ~$1M in the quarter is manageable given the launch investment phase. The company has adequate liquidity to fund EXXUA commercialization through the initial ramp period.
Q&A Highlights
Why are early prescribers choosing EXXUA?
CEO Josh Disbrow highlighted mixed motivations from the first 100+ prescribers :
- Patients not getting robust response on current medications
- Patients experiencing side effects (sexual dysfunction, weight gain) on SSRIs/SNRIs
- Interest in the novel mechanism of action (5-HT1A agonist)
- Ease of prescribing through RxConnect platform with minimal barriers
"Any new drug is gonna often get the problematic patients that have been on countless medications, and so we expect that, but we also expect as time goes on, physicians will probably better identify patients that are perhaps not down to the full extent of having tried many medications and start to position the product perhaps earlier in use."
What triggers sales force expansion?
Management was clear that expansion will be tied to profitability, not external capital :
"There's no plan to expand without cash on hand to support that... The primary trigger with the board approval will be cash flow supporting it. There will not be any appetite to raise capital."
Direct-to-consumer strategy:
The company is pursuing a digital-first DTC approach :
- SEO and keyword search campaigns targeting patients seeking alternatives
- Exploring social media and forums (Reddit, chat rooms) while maintaining FDA compliance
- No traditional over-the-air media spend planned initially
- Focus remains on face-to-face sales force interactions as primary driver
Supply chain confidence:
Management confirmed adequate inventory through calendar year 2026 and beyond, with API and components stateside at the contract manufacturer .
CFO Modeling Guidance
CFO Ryan Selhorn provided key assumptions for modeling :
Important note on revenue recognition: The 14-day no-cost titration pack and guaranteed coverage for months 1-2 mean "scripts will grow ahead of net revenue in the early going." Full month-3 refills begin in June 2026 quarter .
Key Risks & Concerns
1. EXXUA Execution Risk: The success of the entire company strategy now hinges on EXXUA commercial uptake. The $22B MDD market is competitive with established SSRIs and other antidepressants.
2. Legacy Portfolio Decline: Intentional deprioritization of ADHD and Pediatric products means revenue headwinds will continue until EXXUA scales.
3. Cash Runway: While the $30M cash balance provides runway, negative adjusted EBITDA during the launch phase will pressure liquidity if EXXUA adoption is slower than expected.
4. Warrant Liability Volatility: The $27.3M derivative warrant liability creates significant non-cash earnings volatility tied to stock price movements.
5. Limited Analyst Coverage: With a $19M market cap and minimal institutional coverage, price discovery and capital access may be constrained.
Forward Catalysts
The next earnings report will be the first to include a full quarter of EXXUA commercial sales, providing critical visibility into early adoption trends.
Conference Call Details
The company hosted an earnings call on February 3, 2026 at 4:30 PM ET. Key participants included CEO Josh Disbrow and CFO Ryan Selhorn . Analysts from Lake Street (Thomas Flaten), Maxim Group (Naz Rahman), and Ascendiant Capital (Ed Wu) asked questions.
Sources: Aytu BioPharma 8-K filed February 3, 2026; Q2 FY2026 Earnings Call Transcript. Stock data from market data providers.